-
Welcome to Religious Forums, a friendly forum to discuss all religions in a friendly surrounding.
Your voice is missing! You will need to register to get access to the following site features:- Reply to discussions and create your own threads.
- Our modern chat room. No add-ons or extensions required, just login and start chatting!
- Access to private conversations with other members.
We hope to see you as a part of our community soon!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
water and electricity
- Thread starter Eddi
- Start date
Secret Chief
nirvana is samsara
Electricity is not a conductor. Some materials conduct electricity - graphite for example; diamond not so much.Is it something to do with electricity being some kind of conductor?
I meant to say is water a conductor!Electricity is not a conductor. Some materials conduct electricity - graphite for example; diamond not so much.
Will correct post now......
Why is it dangerous to mix water and electricity?
If I'm taking a bath and someone throws an electrical appliance that is connected to the mains into the bath-tub how and why would I die
Is it something to do with water being some kind of conductor?
Pure water is an insulator, but you'll never find pure water in nature. It's all the other stuff, the impurities and charged ions that turn it into a conductor.
So how and why does it kill you if you make electricity go into it?Pure water is an insulator, but you'll never find pure water in nature. It's all the other stuff, the impurities and charged ions that turn it into a conductor.
So how and why does it kill you if you make electricity go into it?
Typically... Your heart is powered by electricity , electricity from external sources interferes with the signals that make your heart beat either stopping your heart or making it beat rapidly or irregularly.
Then why is the electric chair such a slow and inefficient form of execution?Typically... Your heart is powered by electricity , electricity from external sources interferes with the signals that make your heart beat either stopping your heart or making it beat rapidly or irregularly.
It's not meant to be when done right. It depends on body size, voltage, current etc.Then why is the electric chair such a slow and inefficient form of execution?
I think it's barbaric though. Just shoot someone.
Then why is the electric chair such a slow and inefficient form of execution?
^^^ THIS ^^^It's not meant to be when done right. It depends on body size, voltage, current etc.
I think it's barbaric though. Just shoot someone.
I think being shot would be way better than the electric chairI think it's barbaric though. Just shoot someone.
But personally I would want to be finished off by recieving a massive opiate overdose
I think that if we're going to do executions then the individuals getting executed should be able to choose the method, within reason
Heyo
Veteran Member
The reaction to electricity can widely differ between individuals. There are quite some people who have no problem letting electricity pass through their bodies.Then why is the electric chair such a slow and inefficient form of execution?
wellwisher
Well-Known Member
Deionized and distilled water do not conduct electricity. However, water is rarely in the form of pure water. In the case of electricity, and a bathtub of water, the water in the tub is most likely, city water with mineral ions; hardness, as well as ions from the soap, shampoo, shave cream and bubble bath; surfactants. DC or direct current is less harmful than AC or alternating current. The AC current is what is in most households. It operates at 60 cycles per second, like a wave and the beat of the human heart. AC electricity can shift the heart beat out of rhythm. DC is a flat wave and not a sine wave, so it will mostly hurt, but is not as heart attack deadly, all else equal.Why is it dangerous to mix water and electricity?
If I'm taking a bath and someone throws an electrical appliance that is connected to the mains into the bath-tub how and why would I die?
Is it something to do with water being some kind of conductor?
As a college freshman, I did some simple experiments growing plants under electric fields. This was based on research I read about from the 1910's, where strawberries near electric fences would increase sugar content by 20%. A friend of mine, who was a EE or electrical engineer, gave me a DC power transformer, from a sign. It was about 5000 volts and about 1 milliamp. The current was too low to kill you, but the voltage was high enough to hurt like bugger. As a rule of thumb, the electric arc will be 1 inch long for each 10,000 volts. I had about 1/2 inch arc to play with. At first, I played with it to see what would make the best arcs. It turned out to be these circuit based tickets, used for the dorm washers and driers. The circuit ticket would light up and fry, but each demonstration cost me so I stopped.
Living in the dorms at the time, I did my plant experiment on my deck, with one electric plate taped to the ceiling, and the other below plant pot, so I could watch, while I studied my ChemE courses. The experiment drew attention, from all the dare devils in the dorms, who would challenge each other to touch both plates; aluminum foil. It hurt like touching an electric fence, like some of the guys had done before and confirmed. It was funny to watch as they lined up to grab, jump, yell and everyone laugh. I passed on the game, but I did supply the party.
One day, I woke and the top plate, had fallen on my plant, due to the tape on the ceiling having lost its tack. This may have been due to the shock game. The contact with the plant, completed the circuit and electrocuted my plant. The test was over. I realized this set up was dangerous and could have caused a fired as plant dried out.
After that I did some tests electrocuting various wild plants, to watch what would happen. The water in the plants capillary action was broken and the plant wilted in minutes. Plants move potassium ions in their water, so the electric conduction was a full plant wilt effect. After that, I decided to research making a larger pulse device, to take out a tree. Luckily, I lacked the immediate EE skills and aborted. I gave the electric field generator to the next person. That prototype pulse cannon could have been very dangerous, but it did make me realize I was made for R&D.
The AC current is what is in most households. It operates at 60 cycles per second
50 cycles per second in this part of the world.
I've had at least three 240v shocks, most to my hands but one where I knelt on a frayed wire once. Skin resistance is high enough so that these fortunately weren't deadly but if my hands had been wet I suspect things might have been different, and in the bath one has no time to react quickly so as to escape the shock.
Heyo
Veteran Member
I can't remember how many times I got shocked with 220/230 V, but they don't hurt as much as 10,000 V shocks from electric fences.I've had at least three 240v shocks, most to my hands but one where I knelt on a frayed wire once. Skin resistance is high enough so that these fortunately weren't deadly but if my hands had been wet I suspect things might have been different, and in the bath one has no time to react quickly so as to escape the shock.
Usually it's a result of malfunctioning equipment. It's rarely used, and probably not tested thoroughly between uses.Then why is the electric chair such a slow and inefficient form of execution?
FWIW, I find ASMR to be an even slower and more inefficient form of execution.
wellwisher
Well-Known Member
Going back to water and electricity, electrons are held tightly by the oxygen of water; high electronegativity, making water a nonconductor of electrons and electricity. However, the hydrogen protons of H2O are quite mobile; pH affect. This mobility and easy exchange of hydrogen is the basis for the movement of a slower positive type current within water; proton are much heavier than electrons. This positive style of electricity plays a major role in the state we call life.
The hydrogen proton or H+, is the fastest thing in water, even an order of magnitude faster than the small (sodium) Na+ and (potassium) K+ ions that neurons use for transmission of potential; thoughts. The parallel conduction of positive hydrogen current in water is done through the simple exchange of hydrogen bonding hydrogen, within the proton rich matrix of water. Machines use electron based electricity, while life is based on a proton style of electricity; protricity.
Water touches everything in cells and life, and will soft wire; hydrate, all the components for global protricity currents. Water takes this one step further, in that each hydrogen bond in the continuous water matrix; four per water molecule, is like a little binary switch with two settings; polar or covalent; electrostatic or magnetic. Each setting is separated by a small activation energy hill. The result is a unique form of fluid wiring that also has both memory and muscle; size and free energy differences, yet is fluid enough to switch the matrix locally and globally, on command, to balance internal needs with external changes; food, sensory input, etc. The matrix even controls the DNA, so the protein grid is balanced to the real time cellular needs. Organic computing with smart water, self wiring, may be the future.
The hydrogen proton or H+, is the fastest thing in water, even an order of magnitude faster than the small (sodium) Na+ and (potassium) K+ ions that neurons use for transmission of potential; thoughts. The parallel conduction of positive hydrogen current in water is done through the simple exchange of hydrogen bonding hydrogen, within the proton rich matrix of water. Machines use electron based electricity, while life is based on a proton style of electricity; protricity.
Water touches everything in cells and life, and will soft wire; hydrate, all the components for global protricity currents. Water takes this one step further, in that each hydrogen bond in the continuous water matrix; four per water molecule, is like a little binary switch with two settings; polar or covalent; electrostatic or magnetic. Each setting is separated by a small activation energy hill. The result is a unique form of fluid wiring that also has both memory and muscle; size and free energy differences, yet is fluid enough to switch the matrix locally and globally, on command, to balance internal needs with external changes; food, sensory input, etc. The matrix even controls the DNA, so the protein grid is balanced to the real time cellular needs. Organic computing with smart water, self wiring, may be the future.
Twice for me. Neither time was fun. Felt more like a donkey kicking me than electricity, but the aftermath was weird.I've had at least three 240v shocks, most to my hands but one where I knelt on a frayed wire once. Skin resistance is high enough so that these fortunately weren't deadly but if my hands had been wet I suspect things might have been different, and in the bath one has no time to react quickly so as to escape the shock.
wellwisher
Well-Known Member
Let me expand on the concept of water based electricity or protricity and how and why it occurs in water.
Water has an unusually high melting and boiling point for a molecule so light. It is an anomaly of nature. If we compare methane; CH4, ammonia; NH3, water; H2O, and hydrogen fluoride; HF, they all have the same mass or weight per molecule. However, the boiling points, which reflect how self bonded each is, shows a boiling point of -161C for methane, -33C for ammonia, +100C for water and +19C for hydrogen fluoride, even though ammonia, water and hydrogen fluoride all form hydrogen bonds. Water's very high boiling point is way too high for its size, if we draw a line between for these four similar light weight molecules. This is one of the 70 anomalies of water; bucks the trends in other materials.
The reason for this anomaly, is water has two hydrogen bonding hydrogen and two electron pairs for sharing with other water molecules. This allows for up to four hydrogen bonds per water molecule. Ammonia has three hydrogen for hydrogen bonding but and just 1 pair of electrons to share, while hydrogen fluoride has the opposite or one hydrogen bonding hydrogen and three pairs of electrons to share. Water has the perfect balance needed to form extended connections in 3-D, rather than just dimers and trimers like ammonia and hydrogen fluoride.
Water, at the level of hydrogen bonding, is loosely analogous to carbon. Water can secondary bond with up to four entities forming polymers in 3-D. Unlike carbon, where the four bonds are covalent and more or less fixed, the hydrogen bonds of water are more fluid in terms of change and rearranging. This is ideal for smart assembly and the conduction of protricity.
Protricity does not hurt like electricity, because it is slower; hydrogen proton is heavier and slower than an electron; 1837 times more mass. Protricity has a much softer touch, that allows delicate rearrangement of itself and the organics of life, at the nanoscale. With there being 100 times as many water molecules, in a cell, compared to all other molecules combined, water dominates the second bonding arena; smart matrix for protricity.
Since water self binds so strongly, when pure, the foundational assembly of just pure water molecules, is defined as having an activity coefficient =1; by convention. As we add things into pure water, the activity decreases, all the way to an activity coefficient=0. This is water's range of smart wiring, the goal of the wring is to maximize the water's activity as close to 1 as possible by wiring other things to cooperate.
Hydrogen bonding, in general, and in water, in particular, is a very strong secondary bonding force and its maximization within the water leads to the lowest system free energy and leads to the highest residual activity. Any lowering of activity adds free energy to the water and makes it rewire, but in a way to maintain or increase the global activity as high as possible. Essentially water will wire any dissolved moieties and contact surfaces to maximize itself Water is the big fish in this pond and the rest of the little fish need to adapt; water shape shifting the little fish to optimize the wiring and aqueous activity.
One basic example, to better understand self forming water circuits; hydration, is the system of water and oil. If we take olive oil and water and shake we will get an emulsion; lots of small bubbles of both the water and oil. This creates surface tension. We added energy by shaking or agitating, and have placed the system of water and oil into a higher energy state, with the activity of the water driven to way below 1.0. The water is tied up at all the surface area of all the thousands of bubbles. The global 3-D matrix of water will now work to increase the activity again.
If we wait, the oil and water bubbles, will start to coalesce, each its kind, here and there, until we end up with two layers. With the surface contact area with water and oil now minimized, the activity of the water increases back toward 1. Water is the big fish and oil is the school of little fish, due to their comparative liquid state self bonding energies. The needs of water come first and it rewires the emulsion and the oil will follow water's lead, since the oils only uses many van Der Waals bonds, which are much weaker. Oil actually benefits by surface contact with water, but water becomes the big loser, so the big fish takes this back; increases its own activity coefficient. The term hydrophobic is a misnomer since oil does not fear water, other than water will not share for very long, since water is so narcissistic.
If were go back to an emulsion with thousands of tiny bubbles, the surface tension implies stretching instead of compression. Stretching and surface tension in water has to due with the covalent side of the hydrogen bonding binary switch. The covalent bonds stretch out to allow the magnetic overlap of covalent bonding orbitals. Polar is more about change attraction and the compression side of the switch. Pure water has a higher ratio of polar to covalent switches, in the natural state. The oil-water interfaces, by making water touch the oil, messes up the lowest energy state of the water-water binding, and causes the water to assume more of the expanded hydrogen bonding covalent switch setting, since these are more stable at the level of enthalpy; enthalpy offset for the oil surface contact.
The net effects is the emulsion causes water to lose stability, which water tries to compensate with the lower enthalpy covalent switch setting; surface tension. The problem is the surface tension and covalent setting causes water to become too structured, thereby lowering the water entropy; less complex. Water gains one way, but a potential forms in the second law; entropic potential. Water now needs to get away from the covalent switch setting and back to more polar switches; second law driven phase separation. It is a matter of time before the water and oil have to separate; until the entropic potential is gone. Surfactants can cause an emulsion to form with water and oil; suntan lotion. But the second law will eventually break the emulsion; expiration date.
This basic analysis is useful in terms of packing proteins; driven by lowering the surface tension of water to increase entropy. Some proteins are designed to allow residual entropic potential to remain within the water wiring. The protein-water complex wires its self for cyclic catalytic free energy; enzyme.
Let me give a more complex example of progressive smart wiring; series of packing and folding protein wiring steps to become part of an active water-organic complex, wired into the global water; protricity. Below tops is a diagram of a protein, hot off the press; mRNA translation, being surround by water and then folded and packed. The water begin being hydrate the open protein; starts to wire, and then optimizes the wiring; entropy increase for higher complexity of function. This increases the water activity. We end with a more complex version of water and oil.

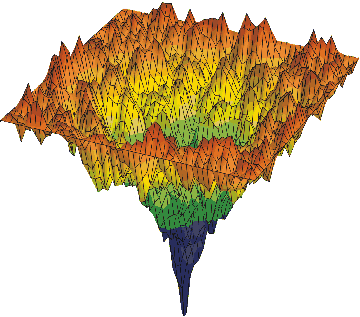
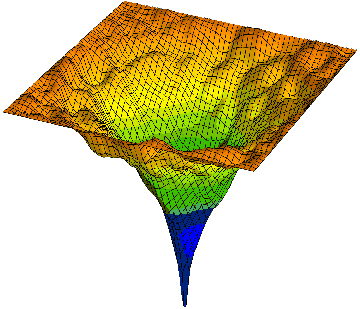
The two diagrams above are called energy landscape diagrams. The reflects the energy that the protein creates in water. The first is connected to the starting protein, all stretched out, with the peaks, the various amino acids along the protein chain exposed to the water. The higher peaks create more surface tension. The second is the folded and packed protein that has been optimized to the water; maximizes water activity with in the global matrix. This is the final wiring diagram.
Unlike a water and simple oil emulsion, where there are more than just two different molecules. This situation is more complex with the different amino acid groups, like attached bubbles of different solvents with different impacts on water, thereby setting urgency; packing priority or folding and packing order. The deep blue represent groups that stabilize the water better than water can do for itself.
The light blue on the right bottom, in the finished protein, appears to be connected to what is called cooperative hydrogen bonding. This is similar to a resonance structure of hydrogen bonding. It is loosely similar to a hydrogen bonding version of benzene. It has many covalent switches, which lower the entropy; more order is induced in the cooperative water. However, cooperative water is very stabilizing in terms of enthalpy; internal energy. It is a unique state of hydrogen bonding, used for storing enzymatic entropic energy. The gain in enthalpy allows entropic potential to be stored; low entropy is possible if enthalpy is dominant. Cooperative hydrogen bonding has the unique property of the first bond cut, no matter where it is in the cooperative, being the strongest bond. This reflects the resonance or group sharing; electrons and protons connections appear to be equal everywhere in the cooperative; all equally strong.
One theoretical way to tap into this stored free energy; entropic potential energy, of the cooperative aqueous hydrogen bonding, is ATP. ATP is used by many enzymes and structural protein, to add catalytic energy for their reactions and actions. The ATP adds or attaches a phosphate group to the enzyme or structural protein, It then picks up a water molecule to forms ADP. The ADP and phosphate are then recycled.
Theoretically, if the water needed to form ADP from ATP was pulled, anywhere the water cooperative; ATP would cut hardest first bond in the cooperative. The impact would similar to a run in a nylon stocking under tension; burst effect as cooperative surface tension relaxes. This could break the cooperative, and create a sudden rise in local entropy; energy vacuum (endothermic entropy increase) that can assistance the enzymatic effect. The net effect would ATP plus the water cooperative, for push and pull up the energy hill. The cooperative wiring would then reset; enthalpy lowers, ready for the next cycle.
Water has an unusually high melting and boiling point for a molecule so light. It is an anomaly of nature. If we compare methane; CH4, ammonia; NH3, water; H2O, and hydrogen fluoride; HF, they all have the same mass or weight per molecule. However, the boiling points, which reflect how self bonded each is, shows a boiling point of -161C for methane, -33C for ammonia, +100C for water and +19C for hydrogen fluoride, even though ammonia, water and hydrogen fluoride all form hydrogen bonds. Water's very high boiling point is way too high for its size, if we draw a line between for these four similar light weight molecules. This is one of the 70 anomalies of water; bucks the trends in other materials.
The reason for this anomaly, is water has two hydrogen bonding hydrogen and two electron pairs for sharing with other water molecules. This allows for up to four hydrogen bonds per water molecule. Ammonia has three hydrogen for hydrogen bonding but and just 1 pair of electrons to share, while hydrogen fluoride has the opposite or one hydrogen bonding hydrogen and three pairs of electrons to share. Water has the perfect balance needed to form extended connections in 3-D, rather than just dimers and trimers like ammonia and hydrogen fluoride.
Water, at the level of hydrogen bonding, is loosely analogous to carbon. Water can secondary bond with up to four entities forming polymers in 3-D. Unlike carbon, where the four bonds are covalent and more or less fixed, the hydrogen bonds of water are more fluid in terms of change and rearranging. This is ideal for smart assembly and the conduction of protricity.
Protricity does not hurt like electricity, because it is slower; hydrogen proton is heavier and slower than an electron; 1837 times more mass. Protricity has a much softer touch, that allows delicate rearrangement of itself and the organics of life, at the nanoscale. With there being 100 times as many water molecules, in a cell, compared to all other molecules combined, water dominates the second bonding arena; smart matrix for protricity.
Since water self binds so strongly, when pure, the foundational assembly of just pure water molecules, is defined as having an activity coefficient =1; by convention. As we add things into pure water, the activity decreases, all the way to an activity coefficient=0. This is water's range of smart wiring, the goal of the wring is to maximize the water's activity as close to 1 as possible by wiring other things to cooperate.
Hydrogen bonding, in general, and in water, in particular, is a very strong secondary bonding force and its maximization within the water leads to the lowest system free energy and leads to the highest residual activity. Any lowering of activity adds free energy to the water and makes it rewire, but in a way to maintain or increase the global activity as high as possible. Essentially water will wire any dissolved moieties and contact surfaces to maximize itself Water is the big fish in this pond and the rest of the little fish need to adapt; water shape shifting the little fish to optimize the wiring and aqueous activity.
One basic example, to better understand self forming water circuits; hydration, is the system of water and oil. If we take olive oil and water and shake we will get an emulsion; lots of small bubbles of both the water and oil. This creates surface tension. We added energy by shaking or agitating, and have placed the system of water and oil into a higher energy state, with the activity of the water driven to way below 1.0. The water is tied up at all the surface area of all the thousands of bubbles. The global 3-D matrix of water will now work to increase the activity again.
If we wait, the oil and water bubbles, will start to coalesce, each its kind, here and there, until we end up with two layers. With the surface contact area with water and oil now minimized, the activity of the water increases back toward 1. Water is the big fish and oil is the school of little fish, due to their comparative liquid state self bonding energies. The needs of water come first and it rewires the emulsion and the oil will follow water's lead, since the oils only uses many van Der Waals bonds, which are much weaker. Oil actually benefits by surface contact with water, but water becomes the big loser, so the big fish takes this back; increases its own activity coefficient. The term hydrophobic is a misnomer since oil does not fear water, other than water will not share for very long, since water is so narcissistic.
If were go back to an emulsion with thousands of tiny bubbles, the surface tension implies stretching instead of compression. Stretching and surface tension in water has to due with the covalent side of the hydrogen bonding binary switch. The covalent bonds stretch out to allow the magnetic overlap of covalent bonding orbitals. Polar is more about change attraction and the compression side of the switch. Pure water has a higher ratio of polar to covalent switches, in the natural state. The oil-water interfaces, by making water touch the oil, messes up the lowest energy state of the water-water binding, and causes the water to assume more of the expanded hydrogen bonding covalent switch setting, since these are more stable at the level of enthalpy; enthalpy offset for the oil surface contact.
The net effects is the emulsion causes water to lose stability, which water tries to compensate with the lower enthalpy covalent switch setting; surface tension. The problem is the surface tension and covalent setting causes water to become too structured, thereby lowering the water entropy; less complex. Water gains one way, but a potential forms in the second law; entropic potential. Water now needs to get away from the covalent switch setting and back to more polar switches; second law driven phase separation. It is a matter of time before the water and oil have to separate; until the entropic potential is gone. Surfactants can cause an emulsion to form with water and oil; suntan lotion. But the second law will eventually break the emulsion; expiration date.
This basic analysis is useful in terms of packing proteins; driven by lowering the surface tension of water to increase entropy. Some proteins are designed to allow residual entropic potential to remain within the water wiring. The protein-water complex wires its self for cyclic catalytic free energy; enzyme.
Let me give a more complex example of progressive smart wiring; series of packing and folding protein wiring steps to become part of an active water-organic complex, wired into the global water; protricity. Below tops is a diagram of a protein, hot off the press; mRNA translation, being surround by water and then folded and packed. The water begin being hydrate the open protein; starts to wire, and then optimizes the wiring; entropy increase for higher complexity of function. This increases the water activity. We end with a more complex version of water and oil.



The two diagrams above are called energy landscape diagrams. The reflects the energy that the protein creates in water. The first is connected to the starting protein, all stretched out, with the peaks, the various amino acids along the protein chain exposed to the water. The higher peaks create more surface tension. The second is the folded and packed protein that has been optimized to the water; maximizes water activity with in the global matrix. This is the final wiring diagram.
Unlike a water and simple oil emulsion, where there are more than just two different molecules. This situation is more complex with the different amino acid groups, like attached bubbles of different solvents with different impacts on water, thereby setting urgency; packing priority or folding and packing order. The deep blue represent groups that stabilize the water better than water can do for itself.
The light blue on the right bottom, in the finished protein, appears to be connected to what is called cooperative hydrogen bonding. This is similar to a resonance structure of hydrogen bonding. It is loosely similar to a hydrogen bonding version of benzene. It has many covalent switches, which lower the entropy; more order is induced in the cooperative water. However, cooperative water is very stabilizing in terms of enthalpy; internal energy. It is a unique state of hydrogen bonding, used for storing enzymatic entropic energy. The gain in enthalpy allows entropic potential to be stored; low entropy is possible if enthalpy is dominant. Cooperative hydrogen bonding has the unique property of the first bond cut, no matter where it is in the cooperative, being the strongest bond. This reflects the resonance or group sharing; electrons and protons connections appear to be equal everywhere in the cooperative; all equally strong.
One theoretical way to tap into this stored free energy; entropic potential energy, of the cooperative aqueous hydrogen bonding, is ATP. ATP is used by many enzymes and structural protein, to add catalytic energy for their reactions and actions. The ATP adds or attaches a phosphate group to the enzyme or structural protein, It then picks up a water molecule to forms ADP. The ADP and phosphate are then recycled.
Theoretically, if the water needed to form ADP from ATP was pulled, anywhere the water cooperative; ATP would cut hardest first bond in the cooperative. The impact would similar to a run in a nylon stocking under tension; burst effect as cooperative surface tension relaxes. This could break the cooperative, and create a sudden rise in local entropy; energy vacuum (endothermic entropy increase) that can assistance the enzymatic effect. The net effect would ATP plus the water cooperative, for push and pull up the energy hill. The cooperative wiring would then reset; enthalpy lowers, ready for the next cycle.
Last edited:
