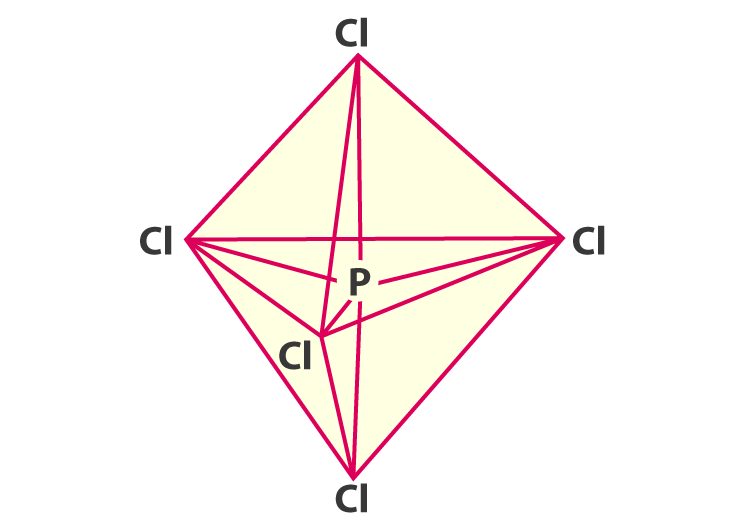
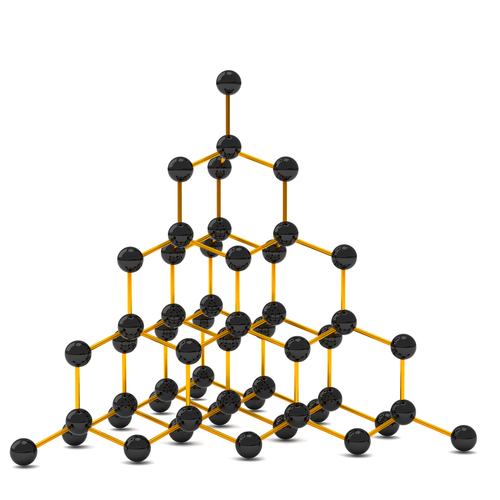
It means that carbon (C) easily bonds to other atoms to create 3-dimensional shapes.
View attachment 41289
...There are an abundance of other elements, which don't do that, and end up looking like:
View attachment 41290
Because with h2o, you can never have a 3-dimensional snowflake, because the molecules are not 3-dimensional -they're pancake shaped, two-dimensional. But with 3-dimensional molecules, stacked on top of each other, we can have things like DNA and trees that can grow in every direction -not like flat pieces of paper.
Hydrocarbon - Three-dimensional structures
No no, I meant what did you mean when you said that seems "mystical"?