I will defend the proposition that the evidence for a very ancient earth (more than 4 billion years) is overwhelming in science and hence, based on the evidence, the only rational conclusion is that the earth is ancient.
Are the Laws of Atomic Physics Constant over time?
The truth that the laws of physics has been constant since the Big Bang, 13.8 billion years ago can be found in the fact of how successfully we can predict the various features of the universe from the processes that occurred just after the Big Bang using our current laws of physics. Specifically the laws of atomic physics tells us what the elemental composition of the universe will be and how that will change as stars make heavier elements in their hot core over the eons. And when we look at the elemental composition in the galaxies, those predictions are matched very well. Thus we have,
1) Laws of atomic physics based on mathematics of quantum mechanics that successfully predict the outcome of experiments (like smashing of atoms) done on earth and by which nuclear power plants, CT scans and nuclear medicine technologies work (LINK)
2) Is also found to predict the elemental and star composition of galaxies and nebulae through nuclear processes that occurred from the early hours of the Big Bang 13.8 billion years ago.
The sequence of steps:-
http://www.einstein-online.info/spotlights/BBN_phys
1) the early universe was filled with a hot plasma consisting of radiation and elementary particles. The various ingredients of this mixture were in thermodynamic equilibrium.
By definition, in thermodynamic equilibrium the energy is distributed evenly among all components of a system. For a simple gas, this would mean that, on average, all of the myriads of particles flying around have the same kinetic energy. For systems like the matter content of the early universe, there is an additional aspect: The particles are constantly involved in reactions in which one kind of particle is converted into another, or several other particles.
For such a system, thermodynamic equilibrium at a certain temperature corresponds to definite values for the relative abundances of the different particle species - how many particles of species A there should be, on average, for each particle of another species B. The relative abundances depend on the temperature, and as the temperature changes, so does the particle mix in the early universe.
2)Let's trace the development starting at about a hundredth of a second and ending at three minutes cosmic time. At the beginning of this time period, the universe was filled with a plasma consisting of matter well-known to physics: protons and neutrons in about equal proportions constituting what physicists call baryonicmatter, as well as electrons, their anti-particles (positrons), neutrinos, and photons.....In this particular epoch, the most influential mediating forces responsible for the particle reactions were electromagnetic interactions and interactions via the so-called weak nuclear force (which is responsible for certain forms of radioactive decay)...via the weak nuclear force, protons were continually being converted into neutrons, and vice versa...If we take all these reactions into account, the statistical formula that govern thermodynamic equilibrium give us a ready answer for the particle content of the very early universe, namely that there were about as many protons as neutrons.
3)In the early universe, the external conditions were constantly changing as the universe expanded and cooled down. The particle mixture at a given point in time depended on the race between reactions establishing the temperature-dependent equilibrium and the change of this very temperature due to cosmic expansion....when the temperature had fallen below a hundred billion Kelvin (corresponding to an energy of 10 MeV per particle), things began to change: At this temperature, the reaction rates for weak interactions between neutrinos and the electromagnetic radiation field are so small that the two kinds of matter effectively "decouple" and cease to interact at all. In addition, most of the electrons and positrons annihilated, while the electromagnetic radiation had cooled down too far to produce new electron-positron pairs. The result was a heating-up of the radiation field (but not of the neutrinos, which had decoupled). A slight imbalance in the number of electrons and positrons led to a small surplus of electrons being left behind - those are the electrons we still find in the cosmos today.
4)While, at the beginning of this new epoch, neutrons and protons were still present in ratios of 1 neutron for every 6 protons, which is close to the equilibrium value at this particular temperature, equilibrium could not be maintained. The expansion changed the cosmos much faster than these reactions could keep up equilibrium - just such a race between cosmic expansion and specific reaction rates as was mentioned above: the weak reactions "froze out". As a result, almost the only weak reaction that still took place at a significant rate was the decay of neutrons into the slightly lighter protons, which is in fact independent of temperature..... Fortunately, however, the universe expanded (and cooled) slowly enough to give another type of reaction time enough to occur: reactions in which neutron and protons combined to form light atomic nuclei. The universe entered the phase called Big Bang Nucleosynthesis (often abbreviated to BBN).
5)At the beginning of Big Bang Nucleosynthesis, at a cosmic time of about 1 second, the situation was quite simple: The nuclear reactions occured fast enough to achieve equilibrium, which strongly favours the very light elements like hydrogen and helium and their isotopes deuterium (d), tritium (t), and helium-3. At this time, the temperature of the radiation-matter-plasma was around ten billion Kelvin, corresponding to an average 1 MeV of energy per particle. Nuclear physics and all the reaction rates necessary for the equilibrium calculations are very well known, as energies like this are easily achievable in laboratory experiments with nuclei.
6)
Considering the relevant time scales for the expansion of our universe and for nuclear reactions, it turns out that hardly any protons will have had time to join existing nuclei and transform into neutrons. On the other hand, reactions in which existing protons and neutrons join to form nuclei were fast enough to ensure that all helium-4 nuclei that can form in this way would indeed have formed. Finally, as mentioned above, we know that, at the beginning of nucleosynthesis, the ratio of neutrons to protons was one to seven - seven protons for each neutron.
With this information, the estimate is straightforward: Consider 16 nucleons, of which 2 are neutrons and 14 are protons (this is precisely the near-equilibrium ratio of 1:7). Out of these one can build only one helium-4 nucleus (as each such nucleus consists of two neutrons and two protons). It has an atomic mass of 4. What remains are 12 protons or, put differently, 12 nuclei of hydrogen atoms, each of which has an atomic mass of 1. The mass ratio of helium-4 to hydrogen is therefore 4/12, in other words: by mass, 75% of matter in our universe is hydrogen and 25% is helium-4. This is a rather simple and solid prediction based on no more than equilibrium physics in a well-known temperature regime. There were a few other elements formed in lower concentrations
Testing the predictions
1) The direct observation of the early abundance can be had from the cosmic microwave background radiation, which comes from the hot plasma itself. As shown the predictions match EXACTLY with observations of element abundance in the microwave radiation. The lines are the theory and the circles are the data points. They match beautifully.
WMAP Big Bang Elements Test
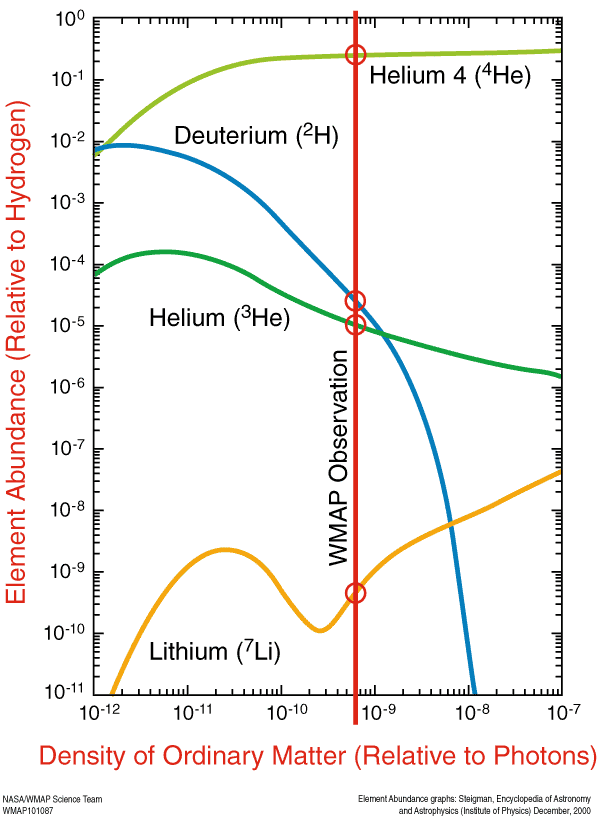
Thus it is seen that the laws governing the atomic decay and fusion of nucleus have remained constant from Big Bang onwards and is seen to work successfully even in the extreme conditions of the early universe demonstrating that we have an excellent grasp of how the law behaves in almost any conceivable environment the universe (or earth) can have since the Big Bang.
Thus the first step is done, I have shown that the laws of physics relevant to radioactivity worked for the last 13.8 billion years and have not changed at all with time.
Are the Laws of Atomic Physics Constant over time?
The truth that the laws of physics has been constant since the Big Bang, 13.8 billion years ago can be found in the fact of how successfully we can predict the various features of the universe from the processes that occurred just after the Big Bang using our current laws of physics. Specifically the laws of atomic physics tells us what the elemental composition of the universe will be and how that will change as stars make heavier elements in their hot core over the eons. And when we look at the elemental composition in the galaxies, those predictions are matched very well. Thus we have,
1) Laws of atomic physics based on mathematics of quantum mechanics that successfully predict the outcome of experiments (like smashing of atoms) done on earth and by which nuclear power plants, CT scans and nuclear medicine technologies work (LINK)
2) Is also found to predict the elemental and star composition of galaxies and nebulae through nuclear processes that occurred from the early hours of the Big Bang 13.8 billion years ago.
The sequence of steps:-
http://www.einstein-online.info/spotlights/BBN_phys
1) the early universe was filled with a hot plasma consisting of radiation and elementary particles. The various ingredients of this mixture were in thermodynamic equilibrium.
By definition, in thermodynamic equilibrium the energy is distributed evenly among all components of a system. For a simple gas, this would mean that, on average, all of the myriads of particles flying around have the same kinetic energy. For systems like the matter content of the early universe, there is an additional aspect: The particles are constantly involved in reactions in which one kind of particle is converted into another, or several other particles.
For such a system, thermodynamic equilibrium at a certain temperature corresponds to definite values for the relative abundances of the different particle species - how many particles of species A there should be, on average, for each particle of another species B. The relative abundances depend on the temperature, and as the temperature changes, so does the particle mix in the early universe.
2)Let's trace the development starting at about a hundredth of a second and ending at three minutes cosmic time. At the beginning of this time period, the universe was filled with a plasma consisting of matter well-known to physics: protons and neutrons in about equal proportions constituting what physicists call baryonicmatter, as well as electrons, their anti-particles (positrons), neutrinos, and photons.....In this particular epoch, the most influential mediating forces responsible for the particle reactions were electromagnetic interactions and interactions via the so-called weak nuclear force (which is responsible for certain forms of radioactive decay)...via the weak nuclear force, protons were continually being converted into neutrons, and vice versa...If we take all these reactions into account, the statistical formula that govern thermodynamic equilibrium give us a ready answer for the particle content of the very early universe, namely that there were about as many protons as neutrons.
3)In the early universe, the external conditions were constantly changing as the universe expanded and cooled down. The particle mixture at a given point in time depended on the race between reactions establishing the temperature-dependent equilibrium and the change of this very temperature due to cosmic expansion....when the temperature had fallen below a hundred billion Kelvin (corresponding to an energy of 10 MeV per particle), things began to change: At this temperature, the reaction rates for weak interactions between neutrinos and the electromagnetic radiation field are so small that the two kinds of matter effectively "decouple" and cease to interact at all. In addition, most of the electrons and positrons annihilated, while the electromagnetic radiation had cooled down too far to produce new electron-positron pairs. The result was a heating-up of the radiation field (but not of the neutrinos, which had decoupled). A slight imbalance in the number of electrons and positrons led to a small surplus of electrons being left behind - those are the electrons we still find in the cosmos today.
4)While, at the beginning of this new epoch, neutrons and protons were still present in ratios of 1 neutron for every 6 protons, which is close to the equilibrium value at this particular temperature, equilibrium could not be maintained. The expansion changed the cosmos much faster than these reactions could keep up equilibrium - just such a race between cosmic expansion and specific reaction rates as was mentioned above: the weak reactions "froze out". As a result, almost the only weak reaction that still took place at a significant rate was the decay of neutrons into the slightly lighter protons, which is in fact independent of temperature..... Fortunately, however, the universe expanded (and cooled) slowly enough to give another type of reaction time enough to occur: reactions in which neutron and protons combined to form light atomic nuclei. The universe entered the phase called Big Bang Nucleosynthesis (often abbreviated to BBN).
5)At the beginning of Big Bang Nucleosynthesis, at a cosmic time of about 1 second, the situation was quite simple: The nuclear reactions occured fast enough to achieve equilibrium, which strongly favours the very light elements like hydrogen and helium and their isotopes deuterium (d), tritium (t), and helium-3. At this time, the temperature of the radiation-matter-plasma was around ten billion Kelvin, corresponding to an average 1 MeV of energy per particle. Nuclear physics and all the reaction rates necessary for the equilibrium calculations are very well known, as energies like this are easily achievable in laboratory experiments with nuclei.
6)
Considering the relevant time scales for the expansion of our universe and for nuclear reactions, it turns out that hardly any protons will have had time to join existing nuclei and transform into neutrons. On the other hand, reactions in which existing protons and neutrons join to form nuclei were fast enough to ensure that all helium-4 nuclei that can form in this way would indeed have formed. Finally, as mentioned above, we know that, at the beginning of nucleosynthesis, the ratio of neutrons to protons was one to seven - seven protons for each neutron.
With this information, the estimate is straightforward: Consider 16 nucleons, of which 2 are neutrons and 14 are protons (this is precisely the near-equilibrium ratio of 1:7). Out of these one can build only one helium-4 nucleus (as each such nucleus consists of two neutrons and two protons). It has an atomic mass of 4. What remains are 12 protons or, put differently, 12 nuclei of hydrogen atoms, each of which has an atomic mass of 1. The mass ratio of helium-4 to hydrogen is therefore 4/12, in other words: by mass, 75% of matter in our universe is hydrogen and 25% is helium-4. This is a rather simple and solid prediction based on no more than equilibrium physics in a well-known temperature regime. There were a few other elements formed in lower concentrations
Testing the predictions
1) The direct observation of the early abundance can be had from the cosmic microwave background radiation, which comes from the hot plasma itself. As shown the predictions match EXACTLY with observations of element abundance in the microwave radiation. The lines are the theory and the circles are the data points. They match beautifully.
WMAP Big Bang Elements Test

Thus it is seen that the laws governing the atomic decay and fusion of nucleus have remained constant from Big Bang onwards and is seen to work successfully even in the extreme conditions of the early universe demonstrating that we have an excellent grasp of how the law behaves in almost any conceivable environment the universe (or earth) can have since the Big Bang.
Thus the first step is done, I have shown that the laws of physics relevant to radioactivity worked for the last 13.8 billion years and have not changed at all with time.